Definitive Guide to Peptide Storage & Stability
Understand the chemistry behind peptide stability and best practices for effective storage
Disclaimer: All information presented in this article is strictly for scientific, academic, and educational purposes. Research peptides discussed here are intended solely for laboratory research and in vitro studies. They are not approved by the FDA or any regulatory agency for human or veterinary use, clinical applications, therapeutic use, or consumption of any kind.
1. Introduction
Proper storage is the single biggest determinant of peptide longevity and experimental reproducibility.
Lyophilization dramatically enhances stability — but it is not permanent protection. Lyophilized peptides are thermodynamically stable yet chemically fragile. The moment atmospheric moisture is absorbed, molecular mobility returns and degradation begins.
This guide provides the laboratory-grade SOP for handling Research Use Only (RUO) peptides — grounded in real chemistry and optimized for researchers who need consistent results. conflict of interest and gives the researcher unbiased, empirical data—not marketing claims.
2. The Chemistry of Degradation
Peptide stability is sequence-dependent. Different residues degrade through different mechanisms. Understanding these pathways explains why proper storage matters.
Primary Degradation Pathways
• Hydrolysis
Water cleaves peptide bonds. Aspartic acid (Asp)—especially Asp-Pro motifs—is particularly hydrolysis-prone.
• Deamidation
Asparagine (Asn) and Glutamine (Gln) convert to Aspartate/Isoaspartate, altering charge and function. Rates increase sharply at alkaline pH.
• Oxidation
Atmospheric oxygen oxidizes electron-rich residues:
– Met → Met-sulfoxide
– Cys → disulfides or sulfinic acids
– Trp/Tyr → oxidative fragments
Oxidation accelerates at pH > 7.
• Photo-Degradation
UV and visible light damage aromatic residues (Trp, Tyr, Phe).
• Diketopiperazine (DKP) Formation
N-terminal X-Pro sequences can cyclize, shortening the peptide.
• Aggregation (Physical, Not Chemical)
Hydrophobic sequences self-associate or form fibrils, especially after freeze–thaw events.
The Rule:
Cold. Dry. Dark.
Every degradation pathway accelerates when any of these three are violated.
3. Lyophilization: What It Protects (and Doesn’t)
Lyophilization removes water by sublimation under deep vacuum, yielding an amorphous solid “cake.”
Benefits
• Suppresses molecular motion → dramatically slows all degradation pathways.
• Halts microbial growth → critical because RUO peptides are not sterile.
• Improves transport stability → sealed vials tolerate room temperature for days/weeks.
What It Does Not Do
It does not prevent degradation once moisture enters the vial. Nor does cake collapse indicate degradation — it is purely cosmetic.
4. Optimal Storage Conditions
Temperature
• Long-term (>1 month): –20°C (or –80°C for sensitive/long peptides).
• Short-term (<1 month): 4°C for unopened vials.
• Do NOT use frost-free freezers: they cycle temperature and destroy stability over weeks due to micro-thawing.
Humidity
• Store vials inside a secondary sealed container with silica desiccant.
• Warm-up rule: always allow the vial to reach room temperature before opening (~20 minutes).
Opening cold creates instant condensation → moisture → degradation.
Light
• Use amber vials or wrap clear vials in foil.
• Even room lights accelerate degradation of Trp-containing sequences.
Freezer Handling
• Avoid repeatedly opening the deep-freezer door.
• Keep vials in a cryo-box to buffer them from temperature swings.
5. Solvent Selection & Reconstitution
Solvent choice dictates stability and solubility. Use the following order of operations.
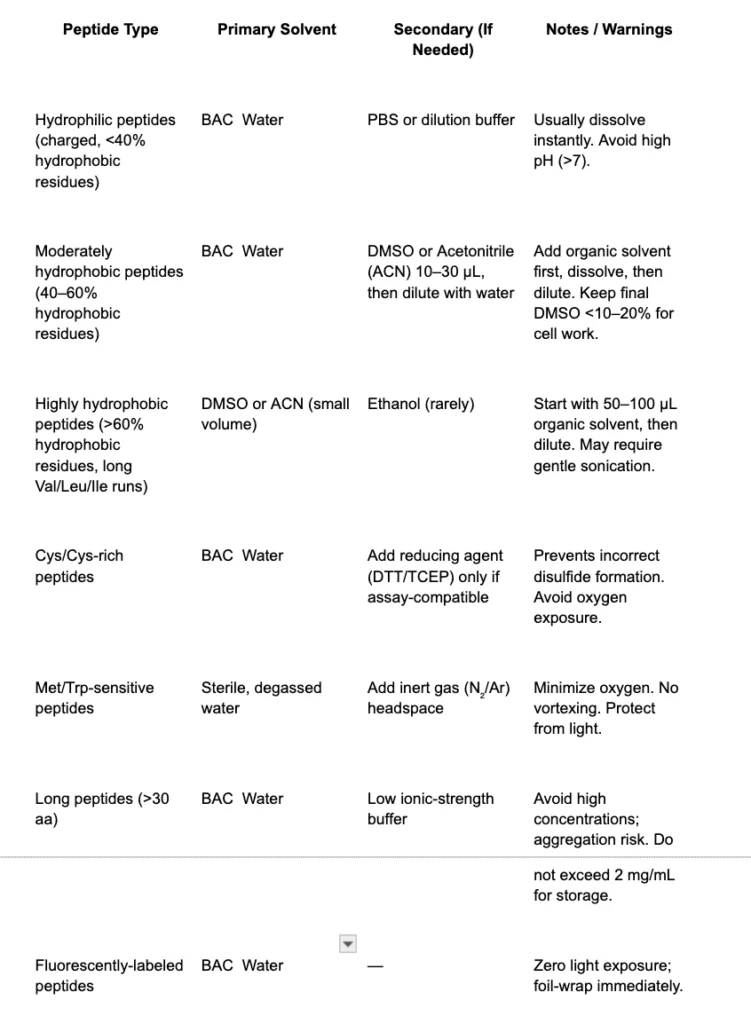
6. Reconstitution SOP (Laboratory-Grade)
Step 1 – Equilibrate
Allow vial to reach room temperature.
Tap/centrifuge to settle powder at the bottom.
Step 2 — Add Solvent
Pipette solvent down the wall of the vial — not directly onto the powder.
Direct jetting causes localized supersaturation and irreversible aggregation.
Step 3 — Dissolve
No vortexing – Vortexing introduces air and shear forces, accelerating oxidation and aggregation.
Correct method:
• Gently swirl
• Tilt and roll
• Mild sonication (10–30 sec) if required
Step 4 — Inspect
Solution must be optically clear.
Cloudiness = precipitation or incomplete dissolution.
7. Freeze–Thaw Damage & How to Avoid It
Freezing creates ice crystal microstructures that mechanically stress peptides.
This is especially harmful for hydrophobic peptides and long sequences.
The Aliquot Rule
- Reconstitute the master vial.
- Immediately portion into single-use aliquots (0.1–1.0 mL).
- Freeze aliquots at –20°C or –80°C.
- Use one aliquot per experiment; never re-freeze.
Additional Notes
• Freeze rapidly, thaw slowly.
• For extremely sensitive peptides: flash-freeze in liquid nitrogen.
• Never freeze high-DMSO (>10%) solutions — mixed-phase freezing damages structure.
8. Special Handling Cases
Oxidation-Prone Sequences
Oxidation-Prone Sequences (Met, Cys, Trp, Tyr)
Use:
• Degassed water
• Inert gas flush (N₂/Ar) before sealing
• Amber vials or foil wrap
• Store at –80°C if long-term
Long Peptides (>30 aa)
These behave like mini-proteins:
• Prone to misfolding
• Aggregate at high concentrations
• Require lower storage concentration (≤2 mg/mL)
Hydrophobic Sequences
Hydrophobic peptides may “oil out” or adhere to glass.
Start with small-volume DMSO or ACN → dilute → sonicate as needed.
Fluorescent Peptides
Zero light exposure from moment of arrival.
9. Summary
Peptide stability is governed by three parameters:
- Temperature
- Moisture
- Molecular mobility.
By controlling these factors — and following proper reconstitution and aliquot protocols — researchers maintain experimental reproducibility and protect COA-verified purity.
10. Related Articles
Explore the rest of our other Pillar Pieces in our Research Hub:
- What are Research Peptides? [LINK HERE]
Learn what research peptides are under the FDA RUO framework, how they differ from GMP/clinical material, and what that means for legality, QC, and lab use.
- The Complete Guide to Research Peptides (RUO) [LINK HERE]
RUO research peptide reference covering RUO framework, manufacturing, HPLC/MS testing, COAs, storage and vendor evaluation in one definitive guide.
- How Are Peptides Made? [LINK HERE]
Step-by-step guide to how peptides are made: SPPS, cleavage, HPLC purification, lyophilization, and QC so you can interpret COAs and compare RUO suppliers.
- How to Read a Peptide COA? [LINK HERE]
Learn how to read a peptide COA the right way—HPLC purity, MS identity, net peptide content, and digital verification—to separate real analytical data from marketing.
- Definitive Guide to Peptide Storage & Stability [LINK HERE]
Practical SOP for peptide storage, reconstitution, and aliquoting. Understand degradation pathways and keep RUO peptides stable and sterile.
- How to Select a Peptide Vendor [LINK HERE]
Tips on how to select a peptide vendor: due diligence checklist for RUO peptides, transparency, COA quality, cold chain, pricing, and marketing red flags.
10. FAQs
Can I reconstitute with sterile water instead of BAC water?
Yes, if it is chemically sterile – distilled water is not a substitute. BAC water contains Benzyl Alcohol which prevents bacterial growth after reconstitution. However, the Benzyl Alcohol is a denaturant and may destabilize sensitive peptides. For these reasons, reconstituted peptides are recommended to be used 4-8 weeks after reconstitution, regardless of solvent.
My peptide arrived warm. Is it ruined?
No. Lyophilized peptides are stable at room temperature for weeks to months provided the seal is airtight
Why is there no expiration date on the vial?
RUO materials generally use retest dates, not expiration dates. A perfectly stored peptide will, theoretically, not degrade.
Can I refreeze a peptide solution?
No, unless flash frozen.
References
Bachem. “Care and Handling of Peptides.” 2021.
Merck KGaA. “Peptide Stability and Degradation Pathways.” 2025.
Sigma-Aldrich. “Solubility Guidelines for Hydrophobic Peptides.” 2024.